Which Subshell is higher in energy
The four most relevant types of subshell are s, p, d, and f. For the same principal quantum number value, an s-type subshell will have the lowest energy and an f-type subshell will have the highest energy of the four, so four electrons which have the same principal quantum number.
Which atomic orbital has the highest energy
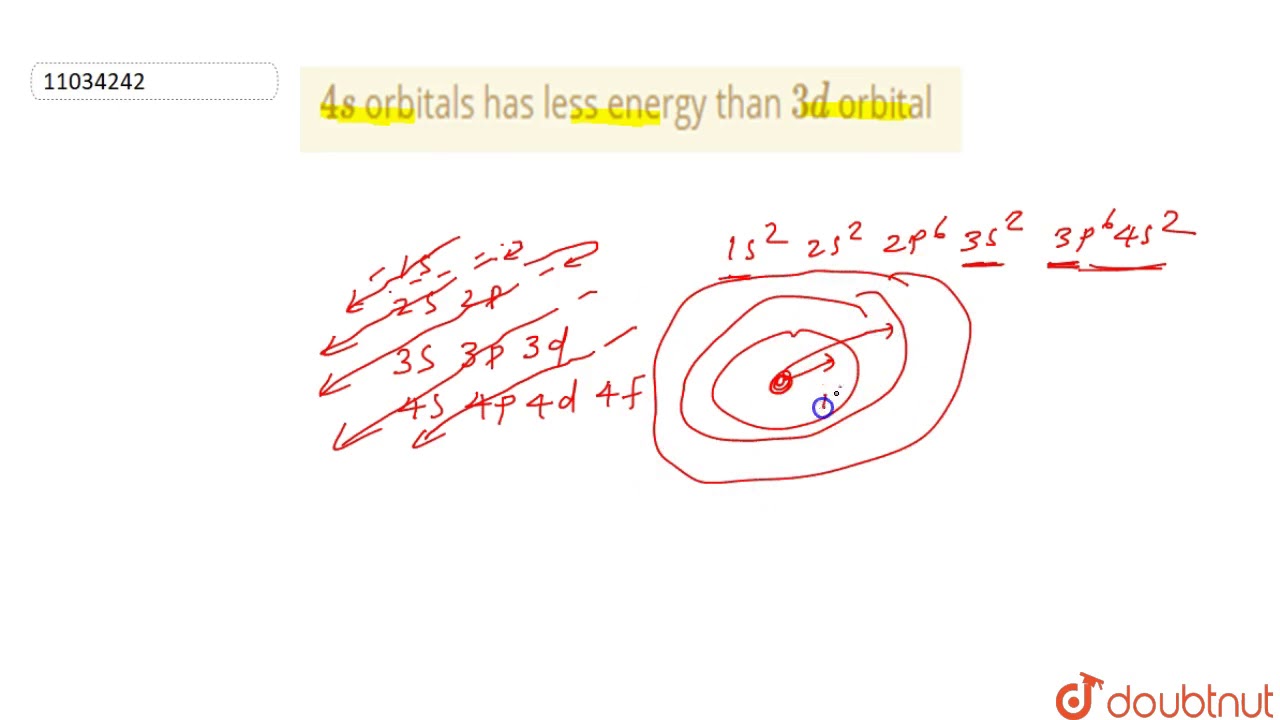
The order of the electron orbital energy levels, starting from least to greatest, is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.
What is the trend in ionization energy
The first ionization energy varies in a predictable way across the periodic table. The ionization energy decreases from top to bottom in groups, and increases from left to right across a period.
What is the N plus L rule
The energy of an orbital depends upon the sum of values of the principal quantum number (n) and the azimuthal quantum number (l). This is called (n + l) rule . According to this rule, ” In neutral isolated atom, the lower the value of (n+ l) for an orbital, lower is its energy.
Why 4s is lower than 3d
The n+l value for a 4s orbital is lesser than the 3d orbital and hence the 4s has lesser energy.
Which has higher energy 4p or 3d
Among 4p, 4s, and 3d orbitals, 3d orbital has the least energy.
Is 5p or 5d higher in energy
Step 1: The energy levels of electron subshells increase in this order from left to right: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p.
Is the s-orbital higher in energy than the D Orbital
The d-orbital is higher in energy than the s- and p-orbitals.
What is the trend in ionization energy in group 4
The ionization energies decrease down the group, although there is a slight increase at lead. The trend exists because: The atoms are getting bigger because of the extra layers of electrons. The farther the outer electrons are from the nucleus, the less they are attracted; therefore, they are easier to remove.
Why is the third ionization higher than the second
The third ionization energy is even higher than the second. Successive ionization energies increase in magnitude because the number of electrons, which cause repulsion, steadily decrease. This is not a smooth curve There is a big jump in ionization energy after the atom has lost its valence electrons.
Why is 4s filled before 3d
We say that the 4s orbitals have a lower energy than the 3d, and so the 4s orbitals are filled first. We know that the 4s electrons are lost first during ionization. The electrons lost first will come from the highest energy level, furthest from the influence of the nucleus.
Which will be of higher energy level between 4p and 3d
Among 4p, 4s, and 3d orbitals, 3d orbital has the least energy.
Do you lose 4s before 3d
In all other respects, the 4s electrons are always the electrons you need to think about first. When d-block elements form ions, the 4s electrons are lost first. The 4s electrons are lost first followed by one of the 3d electrons.
Which is greater 3d or 4p
Even though '3d' and '4p' has equal energies, '4p-' has higher 'n' value (n = 4). So, 3d<4p. According to Aufbau principle '3d' is filled first, then only '4p' will be filled.
Is 4s energy level lower than 3d
The 4s orbital has a lower energy than the 3d, and so fills next. That entirely fits with the chemistry of potassium and calcium.
Is 4d or 5s higher
Even though 5s orbitals have a higher principal quantum number than 4d orbitals, (n = 5 compared to n = 4), they're actually lower in energy. As a result, 5s orbitals are always filled before 4d orbitals.
Is 4d greater than 5p
The order of the electron orbital energy levels, starting from least to greatest, is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. Since electrons all have the same charge, they stay as far away as possible because of repulsion.
Why is 4s orbital lower than 3d
It is according to n+l rule: For 4s n+l = 4+0=4 & for 3d n+l = 3+2 = 5 so 4s has lower energy than 3d orbital.
Why is 3d orbital higher than 4s
3d orbital has greater energy than 4s because it's n+l value is (3+2=5) which is more than n+l value for 4s,(4+0=4) orbital. If two subshells or orbitals have the same n+l value, the subshell or orbital with lower n value will have lower energy.
What is the trend in group 4 of the periodic table
Elements become progressively more metallic down the column. Carbon is especially in its diamond and polyhedral forms is a typical non-metal, silicon is a semiconductor, and tin & lead are typical metals. Although tin has one modification (grey tin) which is isostructural with Ge, Si, and diamond.
Which element in period 4 has the lowest ionization energy
Cesium
Detailed Solution. The correct answer is Caesium. Caesium element has the lowest ionization energy. As we go from Lithium to Cesium down the group, Ionisation potential decreases.
Why is the fourth ionization energy higher than the third
For possible ns2np1 configuration, the removal of fourth electron will be possibly from an inert gas electron configuration. So there will be high jump in the fourth ionisation enthalpy than the third ionisation enthalpy which will take place from ns1 electron configuration.
What is 1st 2nd and 3rd ionization
The third ionization energy is the energy it takes to remove an electron from a 2+ ion. ( That means that the atom has already lost two electrons, you are now removing the third.) And 2nd ionization energy is higher than 1st ionization energy, 3rd is higher than 2nd, and so forth.
Is 3d lower than 4s
The 4s orbital has a lower energy than the 3d, and so fills next. That entirely fits with the chemistry of potassium and calcium.
Why is 4s orbital higher in energy than 3d
Wait, then why after filling the 3d orbitals, the 4s becomes higher in energy level Ans: Once 3d orbitals are occupied by electrons, like in the case of transition elements, because they are closer to the nucleus, they will repel the 4s electrons further away from the nucleus and cause it to have higher energy level.